Harnessing Sensors to Identify Risk in Infants with the EDNA Portfolio
AngelEye’s New Sensor- Based Screening Technology Allows for Early Detection of At-Risk Neonates
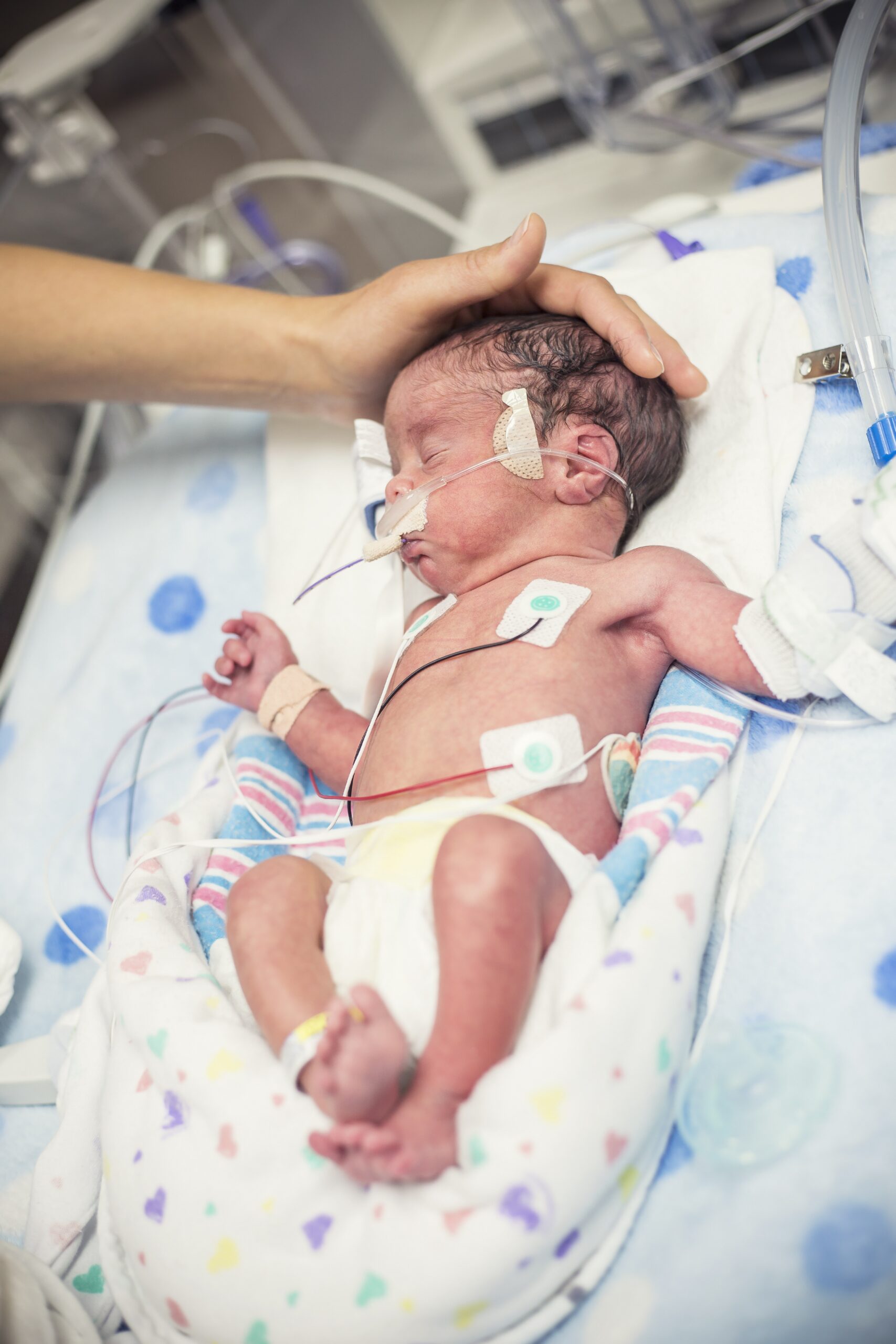
AngelEye Health is pleased to add the Early Detection Neuromotor Assessments (EDNA) portfolio to its suite of solutions. EDNA is dedicated to identifying risk factors in newborns utilizing movement analysis, sensors, clinical experts, and artificial intelligence. This strategic acquisition strengthens AngelEye’s ability to harness sensors at the bedside to help care teams assess, identify, and support early intervention pathways in neonates.



Early Detection Neuromotor Assessments
With nearly 250 hospital partners and technology and almost 10,000 cameras at bedsides, the integration of EDNA’s platform into AngelEye has the unique ability to significantly impact NICU care. EDNA brings the ability to harness sensors at the bedside to help care teams assess, identify, and support early intervention pathways in neonates.
Identifying risks, such as neuromotor, positioning, or sepsis, for example, in neonates, is challenging, which often leads to prolonged hospital stays and long-term complications. Through EDNA’s sensor-based platform, clinicians have more information to understand the neonates’ risks and can better support the baby and their family earlier in the infant’s life, leading to better outcomes.

Built on a Strong Foundation
EDNA’s algorithms and clinical service model have been developed in collaboration with leading scientists and hospitals, including Nationwide Children’s Hospital. Ongoing research is underway to validate further and discover additional sensor-based algorithms and services for newborns.
This page contains information about a medical device product that has not yet received clearance from the U.S. Food and Drug Administration (FDA). The product is currently undergoing the necessary regulatory review process, and its safety and efficacy have not yet been established. Any statements regarding the product’s potential benefits are based on preliminary data and are subject to change upon further evaluation and FDA approval.
